Case Report Published on December 20, 2023
Sarcoidosis Masquerading as Parotid Gland Disorder:
A Unique Clinical Scenario
Sania Mariam Abraham1, Bini Faizal1, Mithun C B2, Smitha N V3
1. Department of Otorhinolaryngology, Amrita Institute of Medical Sciences, Kochi, Kerala;
2. Department of Rheumatology and Clinical Immunology, Amrita Institute of Medical Sciences, Kochi, Kerala;
3. Department of Pathology, Amrita Institute of Medical Sciences, Kochi, Kerala*
ABSTRACT
Sarcoidosis is a multifaceted systemic disorder characterized by granulomatous inflammation, often presenting with diverse clinical manifestations. Parotid gland sarcoidosis is a rare manifestation of Sarcoidosis
This case study presents a 40-year-old woman who presented with bilateral parotid and lacrimal gland swelling and her subsequent journey through diagnostic evaluations, biopsy findings, and treatment insights into the intricacies of diagnosing and managing this complex situation.
Through a detailed exploration of this case, we aim to contribute to the growing body of knowledge surrounding the clinical nuances of sarcoidosis and its diverse array of presentations.
Keywords: Sarcoidosis, Parotid Gland, Granulomatous Parotitis
Introduction
Sarcoidosis is a complex and enigmatic multisystem disorder characterized by the formation of non-caseating granulomas in affected tissues.1 It is seen in young to middle adulthood, occurring between the ages of 20 and 40 years.2 The exact cause of Sarcoidosis is still unknown, and both environmental and genetic factors have been suggested as potential contributors to its development. Recent research show a link between sarcoidosis and the major histocompatibility complex (MHC) region located on chromosome 6p. Variation in this region might impact immune responses, potentially increasing susceptibility to conditions like sarcoidosis.3
While its typical manifestation involves the lungs in 90% of the cases, involvement of skin, lymph nodes, and atypical presentations can pose diagnostic challenges. Lung disease is manifested by bilateral hilar lymphadenopathy and lung parenchymal involvement.4
In Sarcoidosis, parotid gland swelling occurs in less than 6% of patients, and around 40% of these cases exhibit a self-limiting nature.5 It can also affect the lacrimal glands and patients present with symptoms such as xerostomia, keratoconjunctivitis sicca, or salivary gland enlargement.
Given the highly variable nature of sarcoidosis and its multitude of possible disease presentations, treatment plans need to be customized for each patient. Parotid gland sarcoidosis is generally treated with glucocorticosteroids.6
Case Presentation
We report a case of a 40-year-old female who presented to the Otorhinolaryngology department with complaints of bilateral parotid gland pain and swelling with lacrimal gland enlargement of 2 years duration. She reported excessive dryness in her eyes and mouth. The patient has a history of skin lesions over her hands following sun exposure. She also complained of cough and breathlessness.

Figure 1. Clinical photo of patient with bilateral lacrimal and parotid gland swelling
Clinical examination revealed bilateral parotid gland and lacrimal gland swelling (Figure 1). There were also hypopigmented lesions over the skin.
Blood investigations showed elevated levels of serum angiotensin-converting enzyme (SACE) levels of up to 100IU/L. She also tested negative for antinuclear, anti-Ro, and anti-La autoantibodies. Her IgG4 levels were 31.4 which was within normal limits.
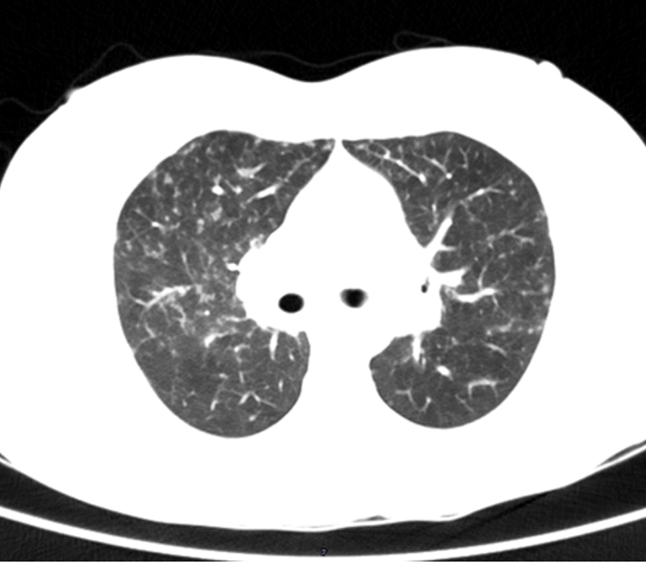
Figure 2. Contrast-enhanced Computerised topography of chest showing, bilateral hilar lymphadenopathy with lung parenchymal peribronchiolar infiltrates
Regional ultrasonography demonstrated features consistent with bilateral sialadenitis involving parotid and submandibular glands. Imaging studies also included a CT chest which showcased miliary nodules and mediastinal lymphadenopathy (Figure 2) Ophthalmology evaluation was done and the Schirmer test yielded a result of 5mm in both eyes suggestive of dry eyes. The differentials we considered were IgG4 glandular disease and Sjogren’s syndrome.
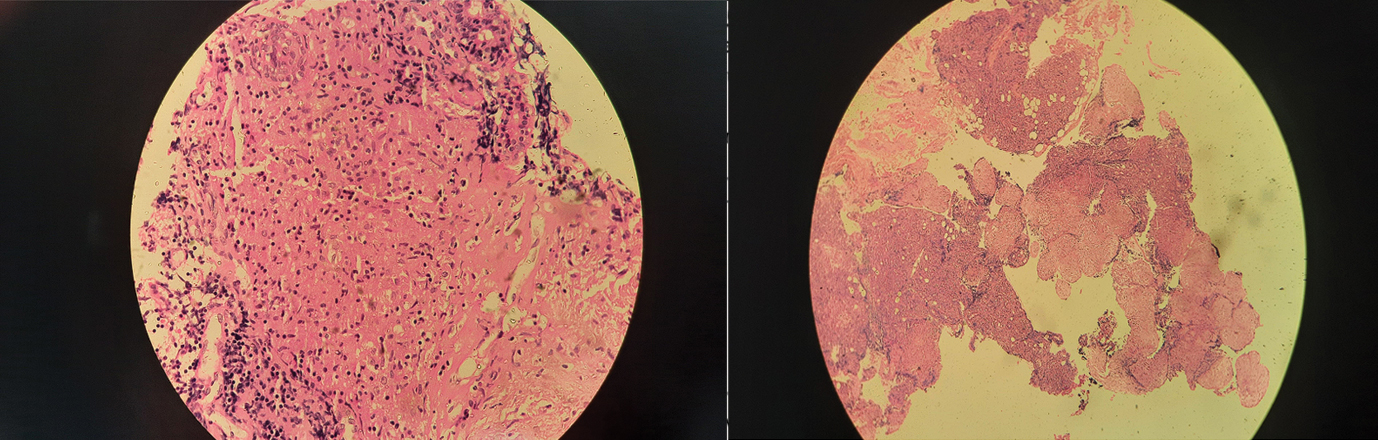
Figure 3. Histopathology specimen of parotid gland shows parotid tissue with non- caseating granuloma
Biopsy from the lip for minor salivary gland and parotid biopsy was done under local anesthesia in the Outpatient department. Histopathological analysis of lip biopsy demonstrated a focus score of less than 1(less than 50 lymphocytes/ focus). Parotid biopsy revealed non-caseating granuloma formed by epitheloid histiocytes, a hallmark of sarcoidosis (Figure 3).
In view of Parotid and lacrimal gland enlargement with bilateral hilar lymphadenopathy, elevated SACE levels, granulomatous inflammation without necrosis on biopsy and a negative Mantoux test, a diagnosis of Sarcoidosis was made. As non-caseating granuloma was seen on biopsy both primary Sjogren’s syndrome and IgG4 glandular-related disease were ruled out.
Treatment and follow-up
The patient was treated with Oral Prednisolone and azathioprine in view of lung parenchymal involvement. Her symptoms improved significantly within 6 weeks of her treatment. Encouragingly, follow-up assessments showed a significant reduction in serum angiotensin–converting enzyme (SACE) levels from 100 to 17 (after a period of 9 months).
DISCUSSION
Sarcoidosis is a complex systemic disorder characterized by the formation of non-caseating granulomas affecting multiple organs and tissues throughout the body. The presentation of parotid gland sarcoidosis can vary. Some patients may experience pain and sicca symptoms while others may be asymptomatic. A rare manifestation of Sarcoidosis is Heerfordt’s syndrome which is characterized by parotid gland enlargement, facial nerve palsy, anterior uveitis and low-grade fever.7 Diagnosis involves a combination of clinical evaluation, imaging studies and biopsy to confirm the presence of non-caseating granulomas. A reliable way to diagnose sarcoidosis is by measuring serum markers. The measurement of serum markers, such as angiotensin-converting enzyme has been considered a reliable diagnostic method for sarcoidosis. ACE is produced by activated macrophages and epithelioid cells and serves as a marker indicative of sarcoidosis. The levels of ACE are linked to the overall granuloma burden in the body. It is also important to note that elevated ACE levels are observed not only in sarcoidosis but also in other granulomatous conditions such as leprosy, histoplasmosis.8 In our case suspicion of Sarcoidosis was raised due to the presence of bilateral hilar lymphadenopathy. The subsequent Chest CT revealed a classic lymphadenopathy pattern known as Garland triad. Another imaging modality which can be used for diagnosis of Sarcoidosis is Gallium-67 Scintigraphy (Ga-67) which shows characteristic panda and lambda signs. Panda sign is radiotracer uptake in bilateral parotid and lacrimal gland with physiological radiotracer accumulation in the nasopharynx and characteristic uptake of Ga-67 in intrathoracic lymph nodes, the shape is of which resembles the Greek letter Lambda is referred to as the Lambda sign.9 There are no characteristic features on B mode ultrasound in patients with parotid gland Sarcoidosis. Some studies show hypoechogenic and hypervascular areas with a heterogenous parenchyma, and no specific findings in other reports.10
Managing sarcoidosis in the parotid gland can be challenging due to the potential impact on facial appearance. Treatment options include systemic corticosteroids to control inflammation and granuloma formation. In cases where corticosteroids are ineffective or have adverse effects, immunomodulatory agents like methotrexate or biological agents such as tumor necrosis factor (TNF) inhibitors may be considered.11
In 2016, Lee et al reported a case of a 49-year-old woman with parotid swelling, which was diagnosed as chronic granulomatous inflammation through Fine needle aspiration cytology (FNAC). The patient underwent parotidectomy and was monitored for symptom recurrence every 6 months.12 Similarly, Hidalgo et al reported a patient with parotid sarcoidosis who also underwent parotidectomy.13
In another study done by Derbel et al a 52-year-old female who initially presented with cutaneous tuberculosis and initiated on ATT (Anti-tuberculosis therapy). Subsequently, she developed parotitis and cervical lymphadenitis and was diagnosed with concurrent Tuberculosis and Sarcoidosis and was treated with oral corticosteroids and was found to have good response.14
In conclusion, Parotid gland sarcoidosis is a rare entity with atypical presentations requiring careful consideration and thorough assessment to clinch the diagnosis. Our case was effectively managed without the need for parotidectomy highlighting the importance of a detailed clinical evaluation involving an efficient multidisciplinary team to manage such cases.
End Note
Author Information
- Dr. Sania Mariam Abraham, MS, Senior Resident Department of Otorhinolaryngology, Amrita Institute of Medical Sciences, Kochi, Kerala
- Dr. Bini Faizal, MS, Professor Department of Otorhinolaryngology, Amrita Institute of Medical Sciences, Kochi, Kerala
- Dr. Mithun C B, DM, MRCP, Associate Professor Department of Rheumatology and Clinical Immunology, Amrita Institute of Medical Sciences, Kochi, Kerala
- Dr. Smitha N V, DNB, Associate Professor Department of Pathology, Amrita Institute of Medical Sciences, Kochi, Kerala
Funding Source: There was no funding from any external agency
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (please name) and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of Interest: None to declare
References
- Ungprasert, Patompong, Jay H. Ryu, and Eric L. Matteson. “Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis.” Mayo Clinic Proceedings. Innovations, Quality & Outcomes 3, no. 3 (September 2019): 358–75.
[Pubmed] | [Crossref] - Lenner, Roberta, Gregory J. Schilero, Maria L. Padilla, and Alvin S. Teirstein. “Sarcoidosis Presenting in Patients Older than 50 Years.” Sarcoidosis, Vasculitis, and Diffuse Lung Diseases: Official Journal of WASOG 19, no. 2 (June 2002): 143–47.
[Pubmed] - Rybicki, B. A., M. C. Iannuzzi, M. M. Frederick, B. W. Thompson, M. D. Rossman, E. A. Bresnitz, M. L. Terrin, et al. “Familial Aggregation of Sarcoidosis. A Case-Control Etiologic Study of Sarcoidosis (ACCESS).” American Journal of Respiratory and Critical Care Medicine 164, no. 11 (December 1, 2001): 2085–91.
[Pubmed] | [Crossref] - Baughman, R. P., A. S. Teirstein, M. A. Judson, M. D. Rossman, H. Yeager, E. A. Bresnitz, L. DePalo, et al. “Clinical Characteristics of Patients in a Case Control Study of Sarcoidosis.” American Journal of Respiratory and Critical Care Medicine 164, no. 10 Pt 1 (November 15, 2001): 1885–89.
[Pubmed] | [Crossref] - “Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) Adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999.” American Journal of Respiratory and Critical Care Medicine 160, no. 2 (August 1999): 736–55.
[Pubmed] | [Crossref] - Diamantopoulos, Panagiotis T., Emmanouil Charakopoulos, Nora-Athina Viniou, Lydia Diamantopoulou, and Maria Gaggadi. “An Unusual Case Report of Unilateral Parotid Gland Sarcoidosis with Spontaneous Remission.” Medicine 98, no. 49 (December 2019).
[Pubmed] | [Crossref] - Fujiwara, Keishi, Yasushi Furuta, and Satoshi Fukuda. “Two Cases of Heerfordt’s Syndrome: A Rare Manifestation of Sarcoidosis.” Case Reports in Otolaryngology 2016 (2016): 3642735.
[Pubmed] | [Crossref]
- Kahkouee S, Samadi K, Alai A, Abedini A, Rezaiian L. Serum ACE Level in Sarcoidosis Patients with Typical and Atypical HRCT Manifestation. Pol J Radiol. 2016 Sep 23;81:458-461.
[Pubmed] | [Crossref] - Yoshimizu T, Suga K, Orihashi N, Soejima K, Kaneko T, Kawamura M, Nakanishi T, Utsumi H, Yamada N. [The appearance of “lambda” and “panda” sign on Ga-67 scintigraphy in sarcoidosis]. Kaku Igaku. 1991 Oct;28(10):1151-7. Japanese.
[Pubmed] - Hofauer, B.; Wiesner, M.; Stock, K.; Peltz, F.; Johnson, F.; Zhu, Z.; Chaker, A.; Knopf, A. Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis. J. Clin. Med. 2022, 11, 2292.
[Pubmed] | [Crossref] - Veena R, Curran J, Blair EA, Sweiss NJ. Salivary glands sarcoidosis. Operative Techniques in Otolaryngology. 2008;19(4):234-6.
[Source] https://www.sciencedirect.com/science/article/abs/pii/S1043181008000742 - Lee DH, Kim JH, Lee JK. Isolated parotid gland sarcoidosis mimicking parotid tumor. J Korean Med Sci 2016;31:644–5.
[Pubmed] | [Crossref] - Hidalgo-López V, Ramos-Murguialday M, Salcedo-Gil CA, et al. Sarcoidosis with bilateral parotid hypertrophy as the first symptom. Rev Espanola Cirugia Oral Maxilofac 2016;39.
- Derbel A, Frikha F, Ghribi M, Salah RB, Bahloul Z. Bilateral Parotid Swelling: Don’t forget Systemic Sarcoidosis!. Ann Clin Cases. 2021; 2(1): 1027
[Source]