Original Research Published on June 28, 2023
A Descriptive Study of Aetiology of Dysphonia in A Voice Clinic
Vishnu Vinayakumar1, Jayakumar R Menon2
1. Fellow in Laryngology and Phonosurgery, Dr Jayakumar’s Laryngology Group, PRS Hospital, Thiruvananthapuram;
2. Director and Senior Consultant, Dr Jayakumar’s Laryngology Group, PRS Hospital, Thiruvananthapuram*
ABSTRACT
Background: Dysphonia is the alteration in normal voice quality due to structural and/or functional causes.1 It can be due to organic pathology that can be a benign cause like vocal nodule or malignancy like Carcinoma glottis. It may also be due to functional causes like muscle tension dysphonia, puberphonia or neurological causes like unilateral vocal cord palsy and spasmodic dysphonia.
Method: This retrospective descriptive study was conducted in all patients who presented with dysphonia in a voice clinic at PRS hospital Thiruvananthapuram in the year 2022. Flexible fibreoptic laryngoscopic examination often supplemented by stroboscopy/ NBI was done in all these patients. The age, sex, and diagnosis of all these patients who underwent laryngoscopic examination for dysphonia were analysed in this study.
Results: Majority of the patients are in the 4th to 7th decade and the most affected age group was 60-69 followed by 40-49. The male: female ratio was around 1.41: 1. Being a tertiary care voice clinic, unilateral vocal cord palsy was found to be the most common cause of hoarseness followed by type I muscle tension dysphonia and spasmodic dysphonia.
Conclusion: Dysphonia can occur due to a variety of causes ranging from unilateral vocal cord palsy to adductor spasmodic dysphonia and from sulcus vocalis to submucous cleft palate. A thorough history taking and clinical evaluation supplemented by endoscopic evaluation with the help of chip on tip flexible laryngoscopy is a must for accurate diagnosis of the aetiology of dysphonia.
Keywords: Unilateral Vocal Cord Palsy, Dysphonia, Spasmodic Dysphonia, Descriptive, Aetiology
Introduction
Dysphonia is the commonest symptom with which a patient attends a voice clinic.
Dysphonia can be classified as 1. Congenital 2. Structural 3. Functional 4. Neurological 5. Joint related 6. Age related 7. Hormone related 8. Psychological 9. Traumatic
Congenital dysphonia includes causes like sulcus vocalis and submucous cleft palate. Structural dysphonia causes include vocal cord polyps, cyst, keratosis, and glottic carcinoma. Functional dysphonias included muscle tension dysphonia and puberphonia. Muscle tension dysphonias can be divided into 4 types.2
MTD 1: Laryngeal isometric (open posterior commissure)/ vocal cord nodules
MTD 2: False vocal cord approximation
MTD 3: Partial anteroposterior compression
MTD 4: Complete supraglottic closure (sphincteric larynx).
Neurological causes of dysphonia include unilateral vocal fold impaired mobility and spasmodic dysphonia. Joint related dysphonias include cricoarytenoid fixation and subluxation. Functional aphonia where the patient present with sudden onset of aphonia is a psychological dysphonia. The other causes of dysphonia include age related, psychological and traumatic dysphonia
Taking a proper history and clinical examination can eliminate a lot of diagnoses and narrow down the focus of our laryngoscopic examination. The core necessity of making a diagnosis of a voice disorder is to be able to successfully visualize the larynx in motion. Flexible fibreoptic laryngoscopy done transnasally is the only method of laryngeal examination that allows visual assessment of vocal function across the dynamic range of voice. Flexible laryngoscopes have the advantage of being less irritating to the patients, possible even in small children and allows visualisation of larynx of patients having exaggerated gag reflex. It can be done even in new born infant and allows the visualization of larynx in physiological position as opposed to the rigid scopy where the larynx is not in the usual position due to the pull on the tongue. This is especially important in professional voice users, where it is important to visualise the vocal cords in physiological position and allows the clinician to do singing laryngoscopic examination for diagnosing dysodia. It also allows evaluation of supraglottic contributions to voice quality in laryngeal dystonia. Nowadays, with the advent of chip on tip cameras for flexible fiberoptic laryngoscopy, quality of the image is at par with that of rigid endoscopy. For all these reasons, flexible fiberoptic laryngoscopic examination with chip on tip camera is the preferred modality for the assessment of aetiology of dysphonia.
Material and Methods
Data was collected retrospectively from the records of patient who had underwent flexible fiberoptic laryngoscopy for dysphonia in the year 2022. Flexible fiberoptic laryngoscopy was done using Olympus system having chip on tip camera. Stroboscopy was done when disorders of Reinke’s space were suspected. Narrow band imaging (I scan) was done to assess for features of malignancy in suspicious keratotic lesions/ growth. Age, sex, and aetiology of patients having dysphonia were noted and entered into Microsoft Excel for statistical analysis.
Results
Out of 268 patients who underwent laryngoscopy for dysphonia, only 111 were females, rest 157 were males. The male: female ratio was around 1.41: 1. The age of patients ranged from 1 year to 87 years. Maximum number of patients belonged to the 60-69 age group (22.39%) followed by 40-49 age group (19.40%) and 50-59 age group (17.91%). The mean age was 46.92 and majority of the males belonged to the 60-69 age group (26.75%) whereas most of the females belonged to the 40-49 age group (26.12%) (Table 1).
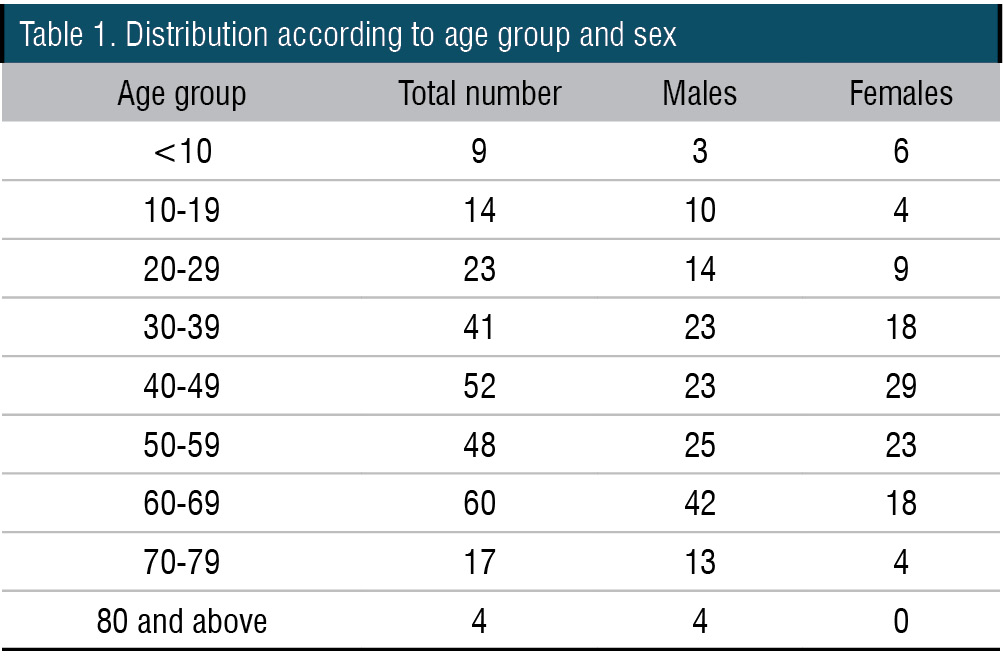
Unilateral vocal fold impaired mobility was the most common cause of dysphonia (17.91%) (Table 2). It included unilateral vocal fold paresis (which may be due to isolated thyroarytenoid palsy) as well as paralysis (Figure 1). One of the patients had isolated interarytenoid palsy. A male preponderance was seen in unilateral vocal fold impaired mobility with 30 out of the 48 patients being males (62.5%). Most of the patients belonged to the age group of 40-49 followed by 60-69 age group.
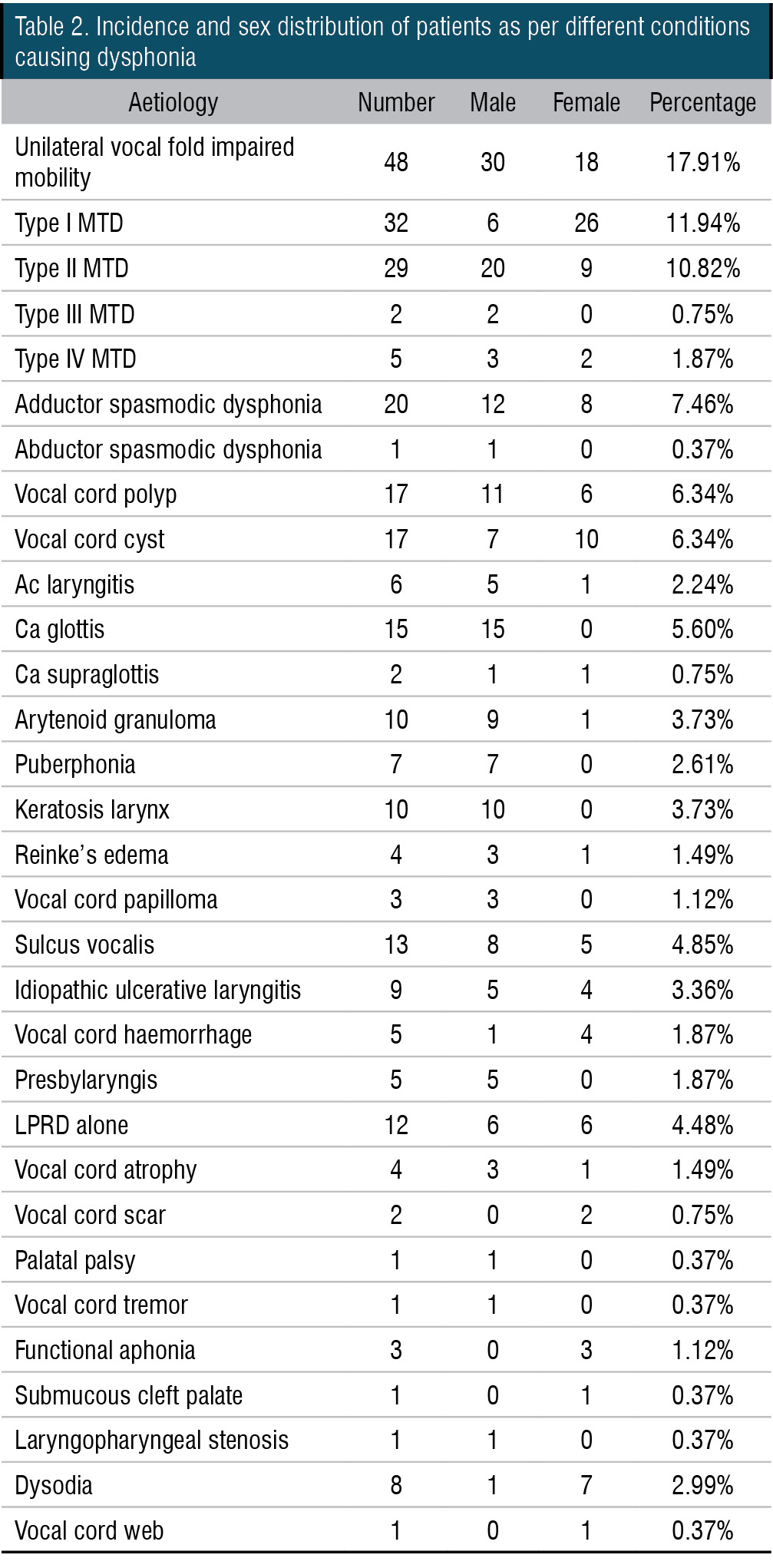
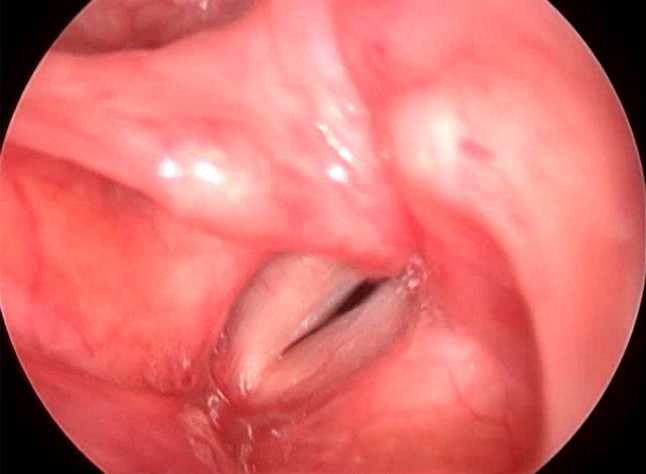
Figure 1. Videolaryngoscopy showing left vocal cord palsy
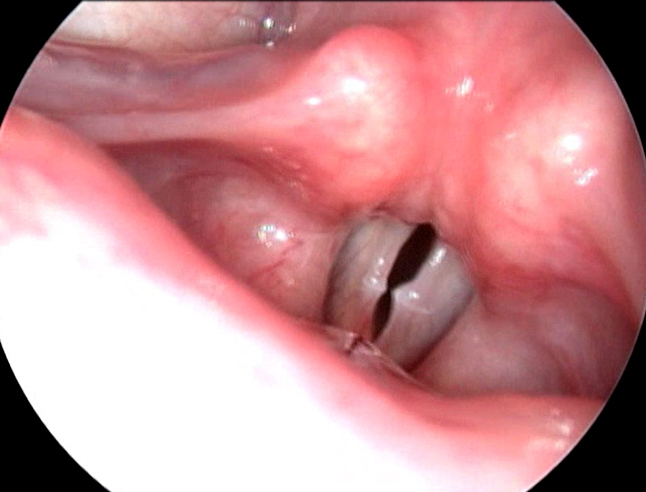
Figure 2. Videolaryngoscopy showing bilateral vocal cord nodules suggestive of type I MTD
Muscle tension dysphonias were the next biggest group in which most patients (11.94%) had type I muscle tension dysphonia with vocal cord nodules (Figure 2). Type II muscle tension dysphonia was seen in 10.82% of the patients (Figure 3) (Table 2). Among the patients having type II muscle tension dysphonia, only 5 were having primary muscle tension dysphonia, rest 24 were seen as secondary muscle tension dysphonia developed as compensatory mechanism for vocal cord pathology such as unilateral vocal cord palsy, vocal cord scar, sulcus vocalis and vocal cord atrophy. One patient with puberphonia had developed secondary type III muscle tension dysphonia (Figure 4). One patient had type I and type IV muscle tension dysphonia. Puberphonia was once considered in the classification of muscle tension dysphonias. Puberphonia is a voice disorder where the adolescent fails to undergo voice change at adulthood and speaks in an unusually high-pitched voice. It was seen in 2.61% of the patients with dysphonia.
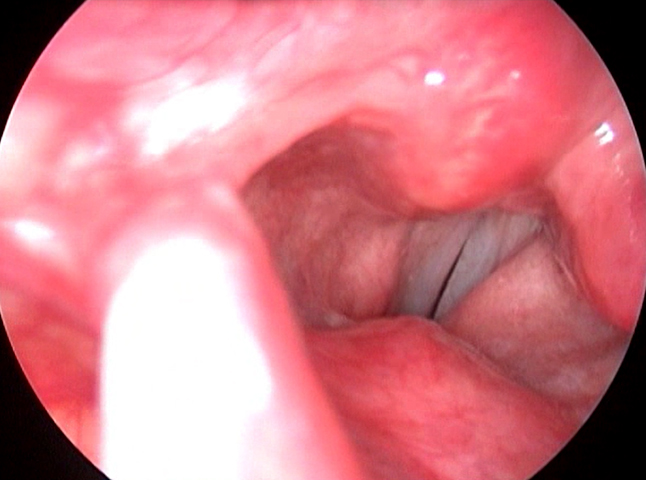
Figure 3. Videolaryngoscopy showing left Type II MTD
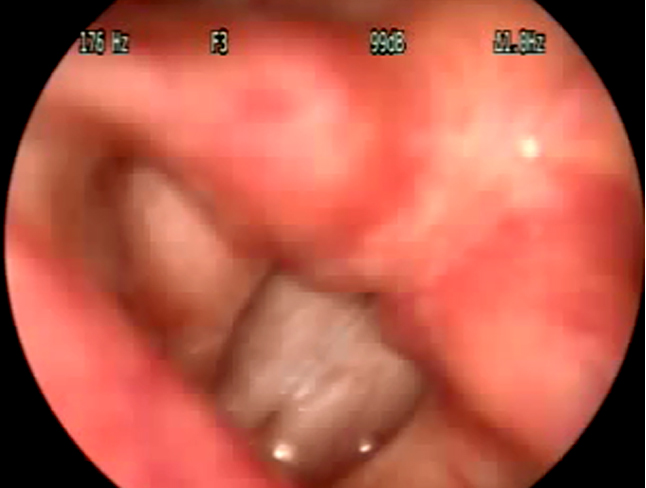
Figure 4. Videolaryngoscopy showing anteroposterior approximation suggestive of Type III MTD
Dysodia is a type of muscle tension dysphonia which is seen only while singing. It can occur due to faulty technique in singing like neck/ clavicular breathing, pharyngeal squeeze, poor oral/ nasal/ throat resonance. Dysodia was seen in 2.99% of the patients. 7 of the 8 patients who had dysodia were females (87.5%) (Table 2).
Adductor spasmodic dysphonia was the cause of dysphonia in 20 patients (7.46%) whereas abductor spasmodic dysphonia was seen only in 1 patient and no patients had mixed spasmodic dysphonia (Table 2).
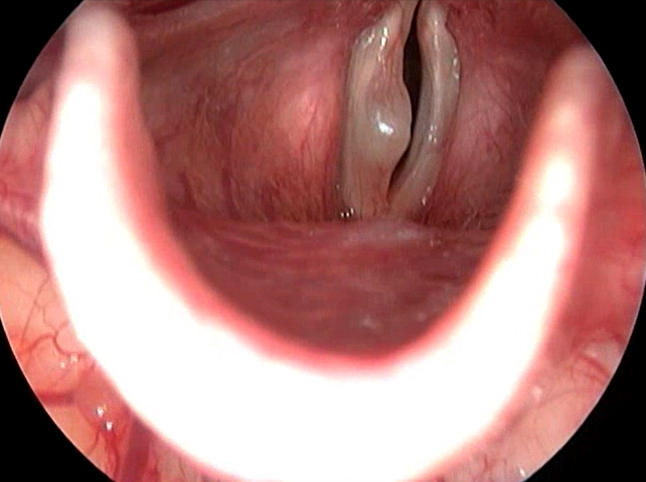
Figure 5. Videlaryngoscopy showing right VC cyst
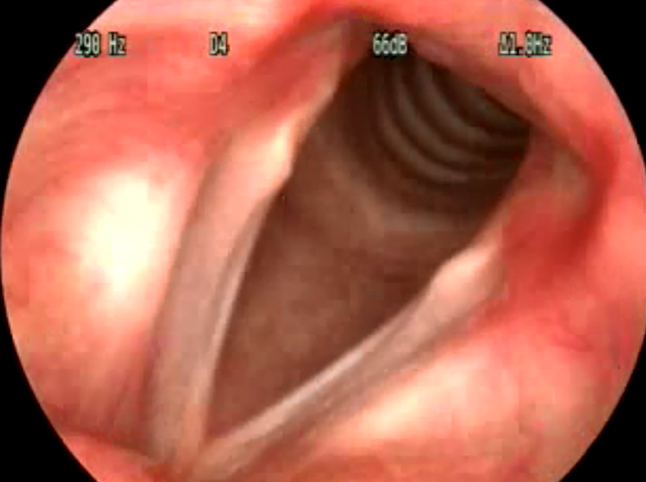
Figure 6. Videolaryngoscopy showing Sulcus vocalis
Vocal cord cyst had the same incidence as vocal cord polyp in this study (Figure 5). It was seen in 17 patients (6.34%). Among the patients having vocal cord cyst, 3 patients had bilateral cyst. One patient had sulcus vocalis along with vocal cord cyst (Table 2).
Malignancy larynx was seen in 17 patients of which only one was in female. 15 of these patients had Ca glottis and all of them were male. 2 patients had Ca supraglottis, of which one was male and the other was female (Table 2).
Sulcus vocalis was seen in 4.85% of the patients who attended the voice clinic (Figure 6). 61.54%
of the patients were males while 38.46% were females (Table 2).
LPRD was seen along with IUL in 8 of the 9 patients and in all patients with arytenoid granuloma (Table 2). LPRD alone was seen to be the cause of dysphonia in 8 of the 29 patients who had LPRD.
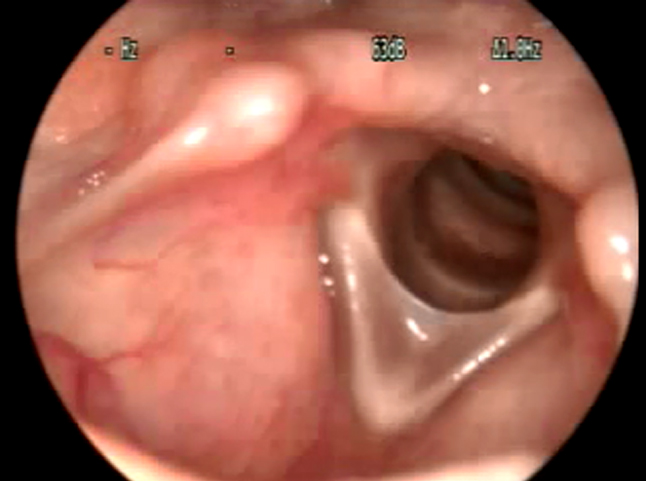
Figure 7. Videolaryngoscopy showing Anterior glottic web
Laryngopharyngeal stenosis was seen in one patient following trauma. 2 patients had submucous haemorrhage after trauma while in 3 others it occurred spontaneously following an upper respiratory infection (Table 2). Vocal cord web was seen in only patient having dysphonia (Figure 7). This occurred following surgical trauma.
There were 3 patients with functional aphonia and all 3 of them were females. Vocal cord scar was seen in 2 patients and both were females. There were 5 cases of presbyphonia which showed key hole phonatory gap on laryngoscopic examination due to atrophic vocal cords. There were 4 other causes of vocal cord atrophy.
There were also rare causes of dysphonia like palatal palsy and submucous cleft palate which resulted in a hypernasal voice in this series. This highlights the importance of resonance in voice.
Discussion
Out of 268 patients who underwent laryngoscopy for dysphonia, only 111 were females, rest 157 were males. The male: female ratio was around 1.41: 1. Male preponderance was seen in most studies. In the study by B.S.S et al though the male: female ratio was 4: 1.3 According to Ghosh et al the sex incidence was more in male (56%) than in females (44%). Baitha et al observed M:F ratio of 2:1.4 Other studies by Deshmukh, Parikh and Batra also showed male preponderance.5-7 The age of patients ranged from 1 year to 87 years. Maximum number of patients belonged to the 60-69 age group (22.39%) followed by 40-49 age group (19.40%) and 50-59 age group (17.91%). The mean age was 46.92 and majority of the males belonged to the 60-69 age group (26.75%) whereas most of the females belonged to the 40-49 age group (26.12%). In the study by B.S.S et al, majority of the patients were in 30-79 age group (57.1%) and the predominant age group was 50-59 years (20.7%).3 In the study by Baitha et al, majority of the patients belonged to the age group of 21-50 years (61.81%) and most of them belonged to the 4th decade of life (21.18%). The age of patients ranged from 6 years to 71 years with a mean age of 40.4 years.4
Unilateral vocal fold impaired mobility was the most common cause of dysphonia (17.91%). According to British literature, incidence of recurrent laryngeal nerve palsy ranges from 1.5% to 14%.3 In the study by Adamu et al, the incidence of unilateral vocal cord palsy was only 5.6%.8 14% of the cases were due to unilateral vocal cord palsy caused by recurrent laryngeal involvement in a study by Somashekara et al.9 In this study, 4% of the patients had bilateral vocal cord palsy and 2% of the patients had superior laryngeal nerve palsy to account for the dysphonia.9 In the study by B.S.S et al the incidence of unilateral vocal cord palsy was 7.1%.3 Since ours is a referral tertiary centre for treatment of all laryngeal pathology, most patients who were undiagnosed for their cause of dysphonia or those who needed further evaluation and treatment were referred here. In our centre, patients having unilateral vocal cord palsy for less than 6 months duration were treated with transthyroid injection laryngoplasty under flexible fiberoptic laryngscopic visualisation on those patients who were willing for the same and patients having unilateral vocal cord palsy for more than 6 months duration were treated with non-selective reinnervation + fat injection or by medialisation thyroplasty or medialisation thyroplasty with arytenoid rotation. This accounts for unilateral vocal cord palsy being the single most cause of dysphonia in this study.
In our study, type I muscle tension dysphonia form the second common cause of dysphonia (11.94%). There was a definite female preponderance with females accounting for 89.66% of the cases in our series. The incidence of vocal nodules was similar (10%) in a study by Adamsu et al. However, females accounted for only 60% of the patients in this series. In most studies, type I muscle tension dysphonia (vocal cord nodules) formed the most common cause of dysphonia followed by vocal cord polyp. Vocal cord nodules were the cause of dysphonia in 30% of the patients in a study by Ghosh et al.10 However, in the study by Soni et al, vocal cord nodules accounted for only 4% of the cases.11 In the study by Mehmet et al, vocal nodules constituted 15.2% of the patients with hoarseness with males constituted 48.86% of the patients and females constituted 51.14% of the patients.12
Type 1 muscle tension dysphonia was followed by adductor spasmodic dysphonia (7.46%) among the commonest causes of dysphonia. Adductor spasmodic dysphonia constituted about 95.24% of the patients with spasmodic dysphonia while abductor spasmodic dysphonia constituted 4.76%. Most studies indicate the incidence of Adductor spasmodic dysphonia to be around 80%.13 Among people with ADSD, 12 were males (60%) and 8 were females (40%). The only person who had abductor spasmodic dysphonia during the period was a female. In a study by Nerurkar et al, 80% of the patients with spasmodic dysphonia were male and only 20% were females.14 Adler et al., in their study of 270 spasmodic dysphonia patients at the Mayo Clinic (Scottsdale, Arizona, USA), showed a female predominance of 79.2 per cent.15 In the study by Hyodo et al, 19.4% were males and 80.6% were females. In this study, adductor spasmodic dysphonia constituted (90-95%) of the spasmodic dysphonias consistent with the results of our study.
Adductor spasmodic dysphonia is one condition which may be missed by primary care ENT surgeons. It is also referred to our centre for treatment which is offered in the form of Botox injection or Endoscopic Thyroarytenoid myoneurectomy (endoscopic TAM). This may account for the disproportionately high number of patients with adductor spasmodic dysphonia in our centre.
Vocal cord polyp was seen in 6.34% of the patients having dysphonia. There was a male preponderance (64.71%) in patients having vocal cord polyp in this study. In the study by B.S.S et al, the incidence of patients having vocal cord polyp was 14.1% and in the study by Baitha et al it was 4.54%.3,4 Male preponderance was also seen in the study by B.S.S et al (61.54%) and in the study by Singh et al (66.6%).3,5 Vocal cord cyst had the same incidence as vocal cord polyp in this study (6.34%). This may be because being a tertiary voice care centre, vocal cord cysts which are more technically challenging to operate is being referred here.
Puberphonia was seen to be the cause of dysphonia in 2.6% of the patients. In a study by Sivrice et al, puberphonia constituted only 0.3% of the patients having dysphonia. However, among the patients aged between 10 and 18 years, it was the second common cause of dysphonia (15.4%).
Malignancy larynx was seen in 17 of the 268 patients with dysphonia. 15 of them had Carcinoma glottis which constituted 5.60% of the patients with dysphonia and all of them were males. One of the two who had Carcinoma supraglottis was a female. In a study by Kiakojoury et al, only 2.5% of the patients had dysphonia due to malignancy larynx17. However, in a study by B.S.S et al incidence of carcinoma glottis was as high as 31% of the patients with dysphonia. Carcinoma supraglottis caused the dysphonia in 10.8% of the patients in this study.
Laryngeal papilloma was the cause of dysphonia in 1.12% of the patients. In the study by B.S.S et al papilloma was the cause of dysphonia in 2.2% of the patients.3 However, in the study by Singh et al, laryngeal papilloma constituted 90% of all benign tumours.5 This is in accordance with the figures reported by Jones et al.18
Sulcus vocalis was seen in 4.85% of the patients who attended the voice clinic. 61.54% of the patients were males while 38.46% were females. Sulcus vocalis is one diagnosis which was missing in many studies of evaluation of dysphonia probably because of the lack of chip on tip camera and stroboscopy. Another reason why it is missing in many studies is that only a few centres provide the necessary treatment for sulcus vocalis. In our centre, sulcus vocalis was treated with injection of fat harvested from the periumbilical area in the subepithelial space and the paraglottic space. In severe cases, the patient may need medialization thyroplasty. In a study by Ghosh et al, 14% of the patients who attended the voice clinic had sulcus vocalis. Of these, 57% were males and 43% were females.19
Conclusion
Dysphonia can be caused by a variety of causes which may be from all the causes listed above. Vocal cord palsy, type I muscle tension dysphonia and Adductor spasmodic dysphonia (ADSD) were the most common causes found in our study. This sequence of diagnosis may not be truly representative of the spectrum of dysphonia in general population because of the pattern of referral is different for a tertiary care voice clinic when compared to a general ENT clinic. However, it also underlines the fact that neuro laryngological conditions like vocal cord palsy, adductor spasmodic dysphonia and vocal cord tremor are not rare. Hence, a thorough history taking and clinical evaluation supplemented by endoscopic evaluation is a must for accurate diagnosis of the aetiology of dysphonia. This study also gives clue regarding the variable causes of dysphonia which must be kept in mind when a dysphonia case is seen in the outpatient.
End Note
Author Information
- Dr Vishnu Vinayakumar, Fellow in Laryngology and Phonosurgery, Dr Jayakumar’s laryngology group, PRS hospital Thiruvananthapuram
Ph no: 9539391560
Mail id: vvk108harekrishna@gmail.com - Dr Jayakumar R Menon, Director and Senior Consultant, Dr Jayakumar’s laryngology group, PRS hospital Thiruvananthapuram
Mail id: jkrmenon@rediff.com
Conflict of Interest: None declared
Financial support: This research received no specific grant from any funding agency, commercial or not-for profit sector.
References
- Neighbors C, Song SA. Dysphonia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Jul 5].
[Pubmed] - Koufman JA, Blalock PD. Functional voice disorders. Otolaryngol Clin North Am. 1991 Oct;24(5):1059–73.
[Pubmed] - S SB, S J. A descriptive study of aetiopathological evaluation of hoarseness: our experience. International Journal of Otorhinolaryngology and Head and Neck Surgery. 2018 Feb 23;4(2):356–61.
[Crossref] - Baitha S, Raizada RM, Singh AKK, Puttewar MP, Chaturvedi VN. Clinical profile of hoarseness of voice. Indian J Otolaryngol Head Neck Surg. 2002 Jan;54(1):14–8.
[Pubmed] | [Crossref] - Singh D, Banjara H, Mungutwar V, Gupta A. Hoarseness of Voice: A Retrospective Study of 251 Cases. International Journal of Phonosurgery & Laryngology. 2014 Jun 1;1(1):21–7.
[Crossref] - Parikh NP. Aetiological study of 100 cases of hoarseness of voice. Indian J Otolaryngol. 1991 Jun 1;43(2):71–3.
[Crossref] - Batra K, Motwani G, Sagar PC. Functional voice disorders and their occurrence in 100 patients of hoarseness as seen on fibreoptic laryngoscopy. Indian J Otolaryngol Head Neck Surg. 2004 Apr;56(2):91–5.
[Pubmed] | [Crossref] - Adamu A, Kolo ES, Ajiya A, Mahmud A, Shuaibu IY, Nwaorgu OGB. Fibreoptic Laryngoscopic Assessment of Patients with Hoarseness: A Cross-sectional Analysis. J West Afr Coll Surg. 2022;12(2):12–6.
[Pubmed] | [Crossref] - pisrt. A cross-sectional observational study of hoarseness of voice in a tertiary care hospital [Internet]. PISRT. [cited 2023 Jul 5].
[Source] - Ghosh SK, Chattopadhyay S, Bora H, Mukherjee PB. Microlaryngoscopic study of 100 cases of Hoarseness of voice. Indian J Otolaryngol Head Neck Surg. 2001 Oct;53(4):270–2.
[Pubmed] | [Crossref] - Soni S, Chouksey S. A Study of Clinicopathological Profile of Patients of Hoarseness of Voice Presenting to Tertiary Care hospital. Indian J Otolaryngol Head Neck Surg. 2017 Jun;69(2):244–7.
[Pubmed] | [Crossref] - Si̇vri̇ce ME, Yasan H, Mustafa TUZ, Okur E, Kumbul YC, Ercan F. Etiology of dysphonia according to age, gender and seasons. TJHSL [Internet]. 2020 [cited 2023 Mar 21];3(2):9–13.
[Source] - Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998 Oct;108(10):1435–41.
[Pubmed] | [Crossref] - Nerurkar NK, Banu TP. Spasmodic dysphonia: a seven-year audit of dose titration and demographics in the Indian population. J Laryngol Otol. 2014 Jul;128(7):649–53.
[Pubmed] | [Crossref] - ADLER C, EDWARDS B, BANSBERG S. Female predominance in spasmodic dysphonia. J Neurol Neurosurg Psychiatry. 1997 Nov;63(5):688.
[Pubmed] - Hyodo M, Hisa Y, Nishizawa N, Omori K, Shiromoto O, Yumoto E, et al. The prevalence and clinical features of spasmodic dysphonia: A review of epidemiological surveys conducted in Japan. Auris Nasus Larynx. 2021 Apr;48(2):179–84.
[Pubmed] | [Crossref] - Kiakojoury K, Dehghan M, Hajizade F, Khafri S. Etiologies of Dysphonia in Patients Referred to ENT Clinics Based on Videolaryngoscopy. Iran J Otorhinolaryngol. 2014 Jul;26(76):169–74.
[Pubmed] - Jones SR, Myers EN, Barnes L. Benign neoplasms of the larynx. Otolaryngol Clin North Am. 1984 Feb;17(1):151–78.
[Pubmed] - Ghosh S, Bakat B, Gupta A, Das S, Saha C, Roychaudhuri BK. Sulcus Vocalis: Our Experience. International Journal of Phonosurgery & Laryngology. 2018 Jun 1;8(1):36–40.
[Crossref]